With the innovative multi-electron transfer cathode using bromine and iodine, it has achieved energy densities up to 1,200 Wh/L, redefining the possibilities for aqueous batteries and paving the way for safer, more powerful energy solutions.
Traditional lithium-ion batteries, renowned for their high energy density, rely on flammable organic electrolytes, raising concerns about their safety. In contrast, aqueous batteries use water-based electrolytes, which are inherently safer but have historically suffered from lower energy densities due to the limited solubility of electrolytes and reduced battery voltage.
Researchers Prof. Li Xianfeng and Prof. Fu Qiang from the Dalian Institute of Chemical Physics of the Chinese Academy of Sciences introduces a significant advancement in aqueous battery technology. Their research unveils a multi-electron transfer cathode using bromine and iodine, achieving a specific capacity of over 840 Ah/L and an impressive energy density of up to 1,200 Wh/L in full battery tests.
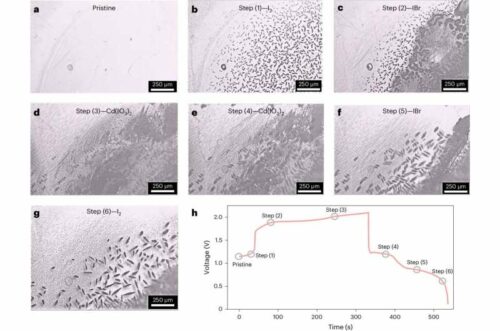
The core innovation lies in the use of a mixed halogen solution containing iodide (I-) and bromide (Br-) ions as the electrolyte. This approach facilitates a multi-electron transfer reaction where I- is converted to elemental iodine (I2) and subsequently to iodate (IO3-). During charging, I- oxidizes to IO3- at the cathode, while the anode manages the flow of hydrogen ions (H+) across the cell. On discharge, these processes are reversed, with IO3- reducing back to I-, and H+ ions moving accordingly to balance the reaction.
A critical aspect of their design is the introduction of bromide ions, which form polar iodine bromide (IBr) during charging. This intermediate reacts with water to generate IO3-, enhancing the kinetics and reversibility of the cell’s electrochemical reactions. During discharge, the reverse reaction occurs, with IO3- oxidizing Br- to bromine (Br2), participating actively in the electrochemical process. The study not only confirms the feasibility of this new cathode through in-situ optical microscopy and Raman spectroscopy but also demonstrates a marked improvement in the energy storage capabilities of aqueous batteries. Prof. Li emphasized the potential of this development to expand the applications of aqueous batteries in power-intensive fields, offering a safer and more efficient alternative to traditional lithium-ion technology. This advancement is poised to redefine safety standards and performance metrics for batteries, providing a robust platform for future innovations in energy storage.







