Researchers have developed Carbonate-superstructured solid fuel cells with hydrocarbon fuels offering high efficiency at lower temperatures.
Although fuel cells work just like batteries based on an electrochemical process, they don’t run down or require recharging. However, the potential advantages of fuel cells are offset by challenges that include cost, performance and durability.
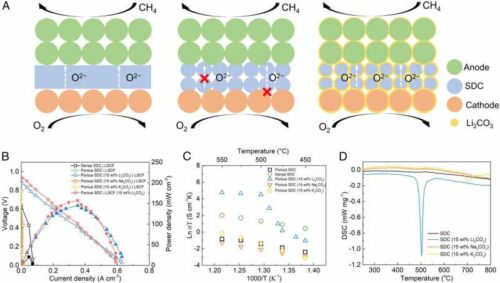
Researchers at Michigan Technological University, took on those challenges, changing the conventional path of a fuel cell by creating an interface between the electrolyte and melted carbonate as an ultrafast channel for oxygen ion transfer. This enabled researchers to invent an entirely new type of fuel cell, a carbonate-superstructured solid fuel cell (CSSFC).
CSSFCs have a wide array of potential uses, from providing energy to operate fuel cell vehicles and home power generation to entire power stations. Because CSSFCs are fuel flexible, they offer higher durability and energy conversion efficiency at lower operating temperatures than other types of fuel cells.
Most fuel cells are powered by hydrogen—typically produced from hydrogen-containing compounds, most often methane—via an expensive process called reforming. But the CSSFC developed in Hu’s lab can directly use methane or other hydrocarbon fuels.
The new fuel cell’s electrochemical performance at lower operating temperatures offers several other advantages. In contrast to the conventional solid oxide fuel cell which usually ranges to 800 degrees Celsius or higher due to very slow ion transfer in a solid electrolyte at a lower temperature, the CSSFC’s superstructured electrolyte can provide a fast ion transfer at 550 degrees Celsius or lower—even as low as 470 degrees Celsius.
The relatively low operating temperature offers high theoretical efficiency and lower cell fabrication costs. Tests on the CSSFC showed an unprecedentedly high open circuit voltage (OCV), which indicates no current leakage loss and high energy conversion efficiency. Researchers estimate that CSSFC fuel efficiency could reach 60%. By comparison, the average fuel efficiency of a combustion engine ranges between 35% and 30%. The CSSFC’s higher fuel efficiency could lead to lower carbon dioxide emissions in vehicles.
Reference : Hanrui Su et al, Carbonate-superstructured solid fuel cells with hydrocarbon fuels, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2208750119