Researchers have developed a solid electrolyte for transporting hydride ions at room temperature, opening doors to safer, more efficient hydrogen-based batteries and fuel cells.
Researchers at the RIKEN Cluster for Pioneering Research in Japan, led by Genki Kobayashi, have achieved a significant milestone in hydrogen-based energy storage. They have successfully created a solid electrolyte capable of transporting hydride ions (H−) at room temperature. This achievement opens up new avenues for the practical implementation of hydrogen-based solid-state batteries and fuel cells, promising enhanced safety, efficiency, and energy density crucial for advancing towards a viable hydrogen-based energy economy. The details of their breakthrough have been published in the prestigious journal Advanced Energy Materials.
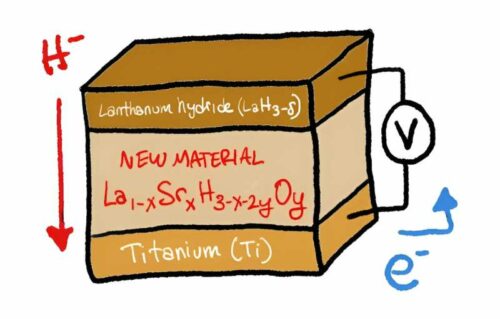
Hydrogen-based energy storage and fuel systems have long been recognised as promising solutions, but they face significant challenges, including safety concerns and efficiency issues. Current hydrogen-based fuel cells rely on proton conduction through a polymer membrane, necessitating constant hydration to prevent drying out. This requirement adds complexity and cost to battery and fuel cell designs, hampering the transition to a hydrogen-based energy economy. To address this challenge, scientists have been developing a means of conducting negative hydride ions through solid materials, particularly at room temperature. The team led by Genki Kobayashi focused their efforts on lanthanum hydrides (LaH3-δ) due to their favorable properties, including easy hydrogen release and capture, high hydride ion conduction, and stability at temperatures below 100°C.
The main hurdle was the fluctuation in the number of hydrogens attached to lanthanum at room temperature, preventing efficient conduction—a phenomenon known as hydrogen non-stoichiometry. To overcome this obstacle, the researchers substituted some of the lanthanum with strontium (Sr) and introduced a small amount of oxygen. This resulted in a formula (La1-xSrxH3-x-2yOy) that enabled efficient hydride ion conduction. The team prepared crystalline samples of the material through ball-milling and annealing processes, demonstrating high rates of hydride ion conduction at room temperature. Moreover, they tested the material’s performance in a solid-state fuel cell, achieving complete conversion of titanium to titanium hydride (TiH2) with an optimal strontium content of at least 0.2. This breakthrough minimizes hydride ion wastage.
In the long term, this development is poised to revolutionize batteries, fuel cells, and electrolytic cells powered by hydrogen, marking a significant turning point in the field. Moving forward, the researchers aim to enhance performance and create electrode materials capable of reversible hydrogen absorption and release, a crucial step towards enabling recharging of batteries and efficient hydrogen storage, essential for the widespread adoption of hydrogen-based energy solutions.