Over the years, innovators have been looking at ways and means of converging digital and medical technologies for creating newer diagnostics and solutions for the healthcare industry. Changes and advancements in the digital industry have opened new vistas for the healthcare industry, providing them tools that may be re-designed and re-engineered to come up with better and more-cost-effective solutions. Imaging and diagnostics is one space within the healthcare industry that is witnessing a number of innovations. Delhi-based J Mitra & Company is one such innovator that has been focusing on creating small-footprint, cost-effective and mobile solutions. Their latest invention, the iQuant Analyzer, addresses the shortage of low-cost medical test infrastructure in local diagnostic centres.
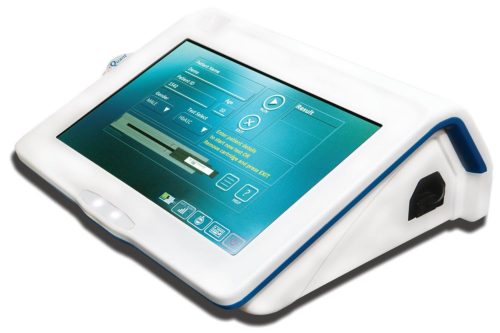
Addressing the gaps in medical facilities
The dearth of low-cost medical instruments often renders local medical facilities incapable of providing quick test reports and medications to patients. Those which have the facilities, are faced with severe restrictions and limitations. Firstly, the tests become expensive due to high cost and high investment of the diagnostic machines. Moreover, restricted availability of such facilities causes semi-urban and rural areas to lack proper delivery of timely aid to patients. Time consuming procedures result in delays of up to 48 hours for generating reports. Lack of portable machines force patients to travel to fixed locations. Complexity of the machines require skilled personnel to run the tests, and requirement of 24×7 electricity for operations lead to higher operational costs for the medical facilities. These challenges intrigued the J Mitra team to design iQuant.
Jatin Mahajan, MD, J Mitra, says, “My father, Lalit Mahajan, and I are research and innovation-driven people. We constantly deliberate on how we can make our solutions more affordable and accessible. Recognising the various shortfalls in our diseases detection and diagnostics system, we decided to work towards a solution that would reach out to the masses rather than have the masses reach out to it.”
Mahajan and team prepared their business blueprint and reached out to Healthcare Technology Innovation Centre (HTIC), a multi-disciplinary R&D centre initiated jointly by Indian Institute of Technology Madras (IIT-M) and Department of Biotechnology (DBT), Government of India. They collaborated to create a point-of-care diagnostic instrument that could read quantitative rapid test kits and provide a numerical value of the test results in less time. R&D and design by J Mitra and HTIC led to the creation of iQuant.
They introduced the beta version of iQuant in December 2017 to test the product and evaluate the Indian market. The official launch took place in August 2018. Currently iQuant supports eight diagnostic tests that includes – TSH (Thyroid Stimulating Hormone), T3 (Triiodothyronine), T4 (Thyroxin), Vitamin D, Dengue NS1 Antigen, Dengue IgM, Dengue IgG and HbA1c test, results of which can be made available in 10-45 minutes.
Target group
From a user/customer perspective, the target group for iQuant are healthcare specialists, medical practitioners and diagnostics solution providers, especially in rural areas, remote locations and settings with limited resources. The product is scaled for small and medium labs, keeping in mind its compact desktop form factor. It allows doctors to quickly diagnose suspected ailments, and as a result, catalysing quicker treatment and recovery.
Mahajan adds, “From an end-beneficiary perspective, it is about reaching out to all those patients and diagnostics-seekers who would find it extremely difficult and prohibiting to receive quality diagnostics solutions in their nearby vicinity. The iQuant Analyzer uses test kits created specifically for Indian conditions.”
How it works
The iQuant Analyzer is an immunofluorescence assay reader, which means, it follows the principle of detecting emission from fluorescent immunoassays. These are simply a different type of immunoassay, where the key variable is the biochemical technique used for detecting the binding of the “detection” antibody and the analyte molecule. The advantages of these test processes lie in higher sensitivity detection of the analyte, simplified reagents and simpler assay designs.
A modern fluorescent based immunoassay uses, as the detection reagent, a fluorescent compound which absorbs light or energy (excitation energy) at a specific wavelength and then emits light or energy at a different wavelength. The difference between the wavelength of the excitation light and the emission light is called the Stokes shift. The greater the shift or difference in the wavelength, the less interference there will be by having the excitation light detected as part of the emission light. Recently several technical improvements, including narrow wavelength low-cost light sources, newer more stable fluorophores with wide Stokes shifts, stable solid-state light detectors and microprocessors that process and analyse the data from each test, have enabled the implementation of a high sensitivity immunoassay system easily.
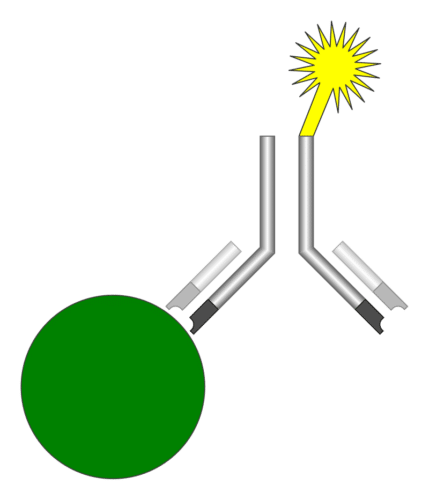
The iQuant Analyzer consists of a lateral-flow immunofluorescence assay test kit-reader (which is also developed by HTIC and J Mitra). It captures the fluorescence created by the chemical reaction occurring on the test kit with the help of a specially designed optics module (which houses optical elements, and photo sensor) and applies necessary calibration information to convert the electrical signal into the final result value.
The advantage of the design lies in its portable, lightweight form factor. It weighs about two kilograms and has a 1-hour battery back-up in case of power failure. Its inbuilt memory stores up to 1-lac patients’ data. A processor unit enables quick report generation. The results can be viewed over a 10-inches intuitive touchscreen display. Data can be transferred to a proprietary cloud facility (iQCloud) or can be printed wirelessly using a Bluetooth printer.
Beating the odds
Building a single system to analyse different kinds of test kits, that too in an ergonomic and economical form, was not an easy task. Mahajan recalls, “When we initially came up with the thought, looking at the number of parameters that we were trying to handle – cost of equipment, cost of test results, size of equipment, portability, ease of handling, scalability, number of tests it could handle etc. – made ourselves sceptical about our ambition. But we already had a very potent ally in HTIC (IIT Madras). Both of us hit the ground from day one.”
The beta launch phase allowed them to further crease out the minor flaws and limitations, enabling them to optimise the solution. The manufacturing of iQuant is being carried out under guidance of HTIC. Mahajan informs, “We face operational challenges due to components which are sourced globally, as there is no ecosystem presently in India.”
They also pay attention to quality stringency with a zero-error tolerance policy. They meet quality requirements through certifications like ISO 9001:2015, EN ISO 13485:2016, WHO-GMP and CE.
Expanding the market
J Mitra is currently serving iQuant to the Indian market only, having sold over 400 units in the first four months since the official launch. “Demands are far exceeding the production currently. So, we will focus on international markets only after we have majorly fulfilled the demands in our country”, says Mahajan. J Mitra already has export channels to over 45 countries across Europe, Latin America, Middle-East, Africa, SAARC, South-East Asia including the USA and UK, which will eventually help them to get the iQuant Analyser to customers abroad.

While there are no immediate upgrades planned for iQuant, future plans include addition of automation steps to further enhance ease of use. J Mitra may also consider increasing the number of test parameters (diagnostics) that iQuant can handle. With a patent for iQuant filed jointly by J Mitra and HTIC, they aim to change the shape of India’s healthcare sector.








dear sir, we are a charitable lab and need machine for thyroid function test as well as glycosylated hb,pl guide us whether this machine is good enough for these tests,
thanks
T3 T4 TSH HBA1C VITAMIN D3 TEST PER TEST COST kitna hoga