A battery layer could make electric cars go farther, charge safer, and last longer, showing the power of lithium metal batteries.
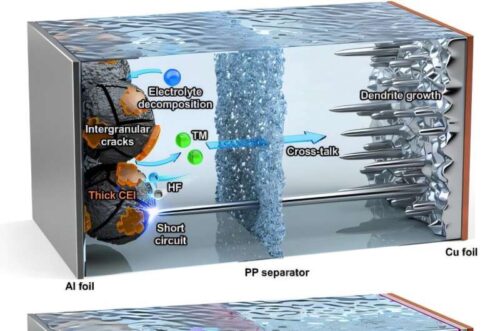
Lithium metal batteries can store about 1.5 times more energy than current lithium ion batteries, potentially increasing electric vehicle range from 400 km to around 700 km per charge. But safety concerns have limited their adoption. During charging, lithium can deposit unevenly on the anode, forming sharp, tree-like dendrites. These needles can pierce the separator between electrodes, causing short circuits, fires, or explosions.
To address this, researchers from POSTECH, Gyeongsang National University, and the Korea Institute of Energy Research (KIER) developed an ultra thin, engineered separator that stabilizes both the anode and cathode. Fluorine F and oxygen O functional groups were added to a standard polyolefin membrane, controlling reactions at the electrode electrolyte interface. A uniform lithium fluoride LiF layer forms on the anode, suppressing dendrite growth, while hydrofluoric acid HF formation at the cathode is prevented, preserving its structure. This single membrane acts as a dual protective layer, stabilizing both electrodes simultaneously.
Under realistic operating conditions, high temperature 55 °C, low electrolyte content, and a thin lithium anode, the batteries maintained 80% of their initial capacity after 208 charge and discharge cycles. Pouch type full cells achieved energy densities of 385.1 Wh kg⁻¹ and 1135.6 Wh L⁻¹, roughly 1.5 to 1.7 times higher than current commercial lithium ion batteries 250 Wh kg⁻¹, 650 Wh L⁻¹.
Molecular level design stabilizes both electrodes while remaining compatible with existing lithium ion manufacturing processes. Computational analyses, including density functional theory DFT and molecular dynamics MD simulations, clarified how functional groups in the separator influence electronic structures and interfacial reactions at the atomic scale.
This technology improves lifespan, safety, and energy density, offering high durability for electric vehicles and large scale energy storage systems, and represents a significant step toward commercializing eco-friendly, high energy batteries.