MIT researchers have created a model that shows how lithium moves in batteries and why it affects charging speed and power.
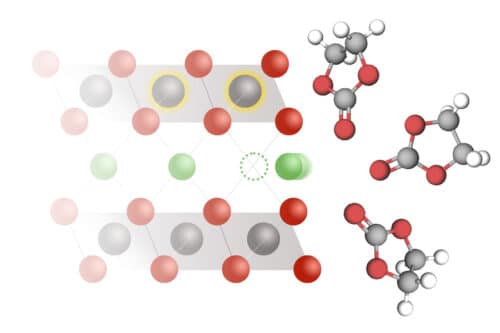
Lithium-ion batteries power everything from phones to electric vehicles, but their performance depends on how fast lithium ions can move into and out of electrodes, a process called intercalation. The speed of this reaction controls how much power a battery can deliver and how quickly it charges. Yet, the detailed mechanism behind it has remained unclear, limiting engineers’ ability to improve battery speed and longevity.
In a recent study, MIT researchers measured lithium intercalation rates across different materials and built a new model to explain how the process is controlled. They found that lithium intercalation is driven by coupled ion-electron transfer (CIET), where a lithium ion and an electron move together into the electrode. This process lowers the energy needed for intercalation, making the reaction easier and more predictable. These insights could help engineers design batteries that charge faster and deliver higher power.
For decades, scientists assumed intercalation speed depended solely on how fast lithium ions moved from the electrolyte into the electrode, following the Butler-Volmer equation, a model nearly a century old. However, experimental measurements often disagreed with this model, varying by as much as a billion-fold for the same reaction.
To address this, the researchers used a technique applying repeated short voltage bursts to an electrode and tested over 50 electrode and electrolyte combinations, including lithium nickel manganese cobalt oxide used in EVs and lithium cobalt oxide common in phones, laptops, and other electronics. The measured rates were much lower than previously reported and did not fit the Butler-Volmer model.
From these experiments, the team proposed the CIET model, showing that an electron must transfer simultaneously with the lithium ion. They also found intercalation rates can be tuned by changing the electrolyte. Different anions reduce the energy needed for lithium-electron transfer, improving efficiency. Using automated experiments and machine learning, they are testing thousands of electrolytes to predict which could improve battery performance.
These findings provide a clearer understanding of lithium flow, enabling faster charging batteries and reducing side reactions that degrade battery life over time.