A pill sized ingestible device that can safely gather small intestine bacteria, improve gut health and open new possibilities for medical research.
Understanding gut health has become critical as research continues to link gut bacteria with digestion, immunity, mood, and chronic diseases. However, studying bacteria in the small intestine remains challenging. Current methods are either invasive, such as endoscopy, or indirect, relying on stool samples that do not accurately reflect microbial activity in the upper digestive tract. This gap has created a strong need for a safe and direct way to study the small intestine microbiome.
To meet this gap, the researchers from IIT Delhi, in collaboration with AIIMS New Delhi, have developed an ingestible device that can collect bacteria directly from the small intestine. The innovation opens new possibilities for gut health research and future clinical applications.
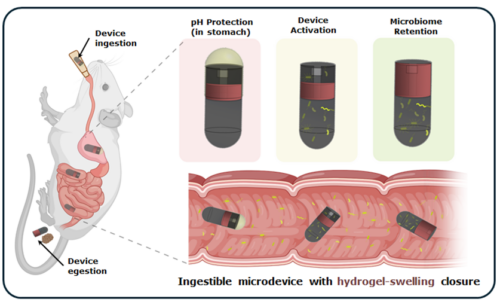
The device is a tiny pill sized microdevice that remains sealed while passing through the stomach. It activates only after reaching the intestine, where it collects bacterial samples and then seals itself again to protect the sample as it moves through the gut. This allows accurate sampling from specific regions of the upper gastrointestinal tract.
The device can collect bacteria directly from the small intestine while remaining closed in the stomach and activating only after reaching the intestine. It uses an enteric coated gelatin capsule to ensure controlled release, enables species level identification of gut microbes, and is designed to operate without causing tissue damage or inflammation. The device is extremely small, with a size comparable to a grain of rice, making it suitable for safe ingestion and sampling.
The technology has been validated in animal studies, showing successful sampling without injury or inflammation. It has led researchers to have a better understanding of the small intestine microbiome and could support early disease detection, monitoring of chronic illnesses, and development of targeted treatments. The team plans to advance this platform technology toward clinical use in India after receiving required regulatory approvals.