Iron batteries are safe and cheap but not very strong. New research could make them store more energy, helping cars, power storage, and future technology.
We want batteries that are low-cost, reliable, and do not cause harm, which is why many of us use iron-based batteries instead of cobalt or nickel, but they do not produce enough power or voltage for electric cars and large energy storage. Still, about 40% of lithium-ion batteries use iron because it is easy to source and avoids issues linked to cobalt mining, such as child labor and unsafe conditions. But the lower performance remains a problem, and most companies work around it rather than solve it. Researchers at Stanford have found a way for iron to store more energy without breaking down, which could help us build batteries that are better suited for future needs.
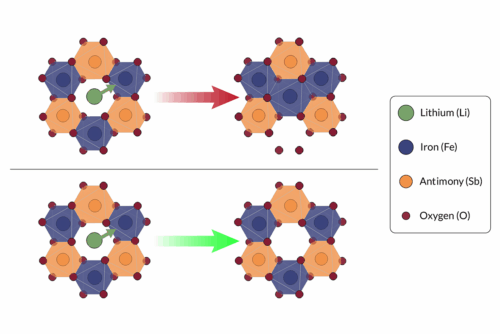
The team tackled this at the atomic level. In most batteries, iron only gives up two to three electrons during charging. If iron could release five electrons instead, batteries would store more energy and deliver higher voltage. Past attempts to do this caused the material to break apart during charging when lithium ions moved away from the cathode. The structure couldn’t handle the stress.
To avoid this collapse, the researchers designed a nanoparticle-based material where iron atoms are spaced out enough to prevent unwanted reactions like oxygen bonding. This spacing allows iron to reach a higher oxidation state and still return to normal during discharge without the material falling apart. The structure flexes slightly rather than cracking, allowing repeated charging cycles.
This approach could increase the energy density of lithium-ion batteries and give iron-based chemistries a performance boost without returning to cobalt or nickel. It also has potential uses in MRI machines, magnetic levitation systems, and even superconductors, technologies that rely on magnetic and electronic properties of materials.
However, turning this lab breakthrough into a commercial product is the next challenge. The team is now optimizing particle shape, material composition, and chemical stability. They are also trying to replace antimony, another expensive and supply-risk element currently in the mix, with something more sustainable.