What if seawater could power your devices—without the high cost? A catalyst made from waste materials could change the future of energy storage.
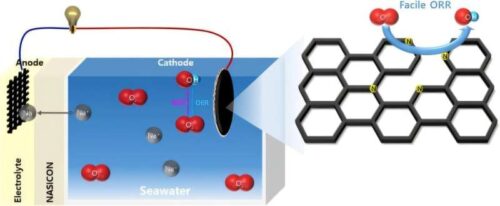
Seawater batteries are poised to be the next frontier in energy storage, offering an efficient way to store and discharge electricity generated from seawater. A crucial hurdle in their commercialization is the development of affordable catalyst materials, a challenge that researchers at UNIST have successfully overcome.
A team of researchers have created a high-performance catalyst for seawater batteries by combining urea with wood waste. This innovative catalyst lowers the overvoltage needed for seawater cells and speeds up the electrochemical reactions, enabling faster electricity discharge.
Traditionally, precious metals like platinum have been used as catalysts, but their high cost makes them impractical for widespread use.
The research team has developed an innovative catalyst using affordable materials—lignin and urea. Lignin, a by-product that makes up 15 to 35% of wood, is produced during the manufacture of paper and biofuels. Urea, which is abundant in industrial wastewater, is rich in nitrogen.
By heating lignin to 800°C while simultaneously reacting it with urea at the same temperature, the team successfully incorporated nitrogen into every part of the lignin structure, creating a high-performance catalyst. This nitrogen doping significantly reduces the energy needed for electricity discharge and replaces specific carbon atoms within the lignin matrix.
When tested in seawater cells, the new catalyst demonstrated performance on par with traditional platinum catalysts. Remarkably, its overvoltage was lower than that of platinum (Pt/C) catalysts.
A lower overvoltage means that more of the stored energy can be efficiently utilized during discharge. The maximum power density achieved by the new catalyst was 15.76 mW/cm², nearly identical to the platinum catalyst’s 16.15 mW/cm²—an important factor for discharge speed.
The team proposed a carbon-neutral approach that not only replaces costly precious metal catalysts but also maximizes the value of biomass and industrial waste. This innovative catalyst has the potential to be applied in various energy storage systems, including metal-air batteries.
Reference: Ji Hwan Hong et al, N-doped carbonized lignin for electrocatalysts in seawater batteries, Chemical Engineering Journal (2025). DOI: 10.1016/j.cej.2025.159219